
Product Characterization Report: CytoShot®
Biological elements present in cord blood, and the potential benefits for use in regenerative medicine.
ABSTRACT
This product characterization report demonstrates the biological elements and quality of StemShot®, an umbilical cord blood derived cellular suspension allograft. The data establishes the total nucleated cell count and cellular viability in post-thaw conditions. Additionally, the surface markers that characterize the cellular material and growth factors present in this cord blood product are presented.
Key Terms
Umbilical Cord Blood Mesenchymal Stem Cells (MSC) Hematopoietic Stem Cells (HSC) CD34, CD45, CD90, CD3, CD14 Growth Factors
VEGF, FGF-2, SCF, IL-1ra, IGF-1 Cytokines
INTRODUCTION
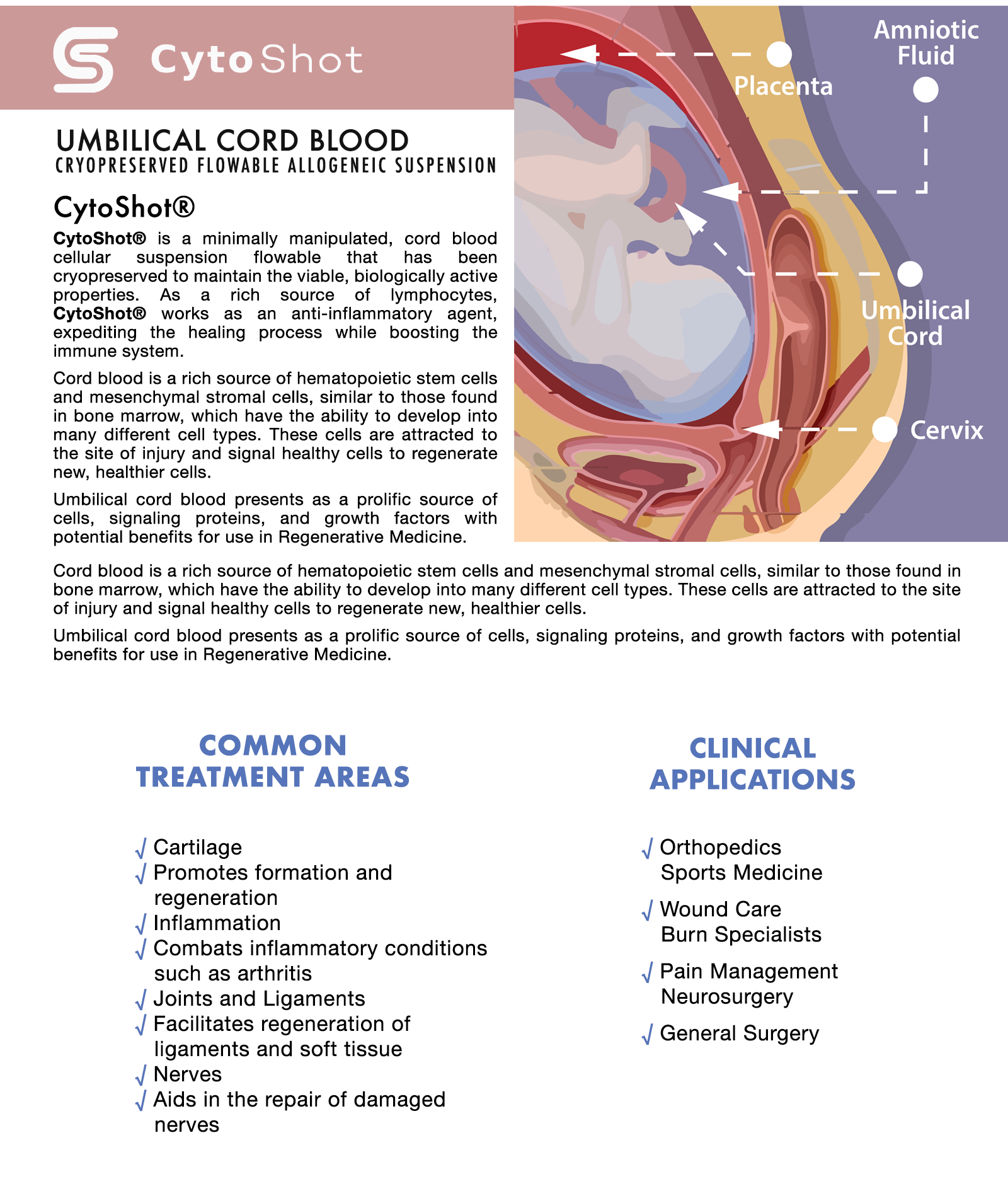
Umbilical cord blood presents as a prolific source of cells, signaling proteins, and growth factors with potential benefits for use in Regenerative Medicine (Damien and Allan 2015). Highly oxygenated blood, rich in nutrients, is transferred from the placenta to the fetus via the umbilical cord. This cord blood is replete with many stem, immune and progenitor cells, in addition to proteins and growth factors that support healthy growth of the fetus (Riordan 2007). Often discarded, umbilical cord blood is now frequently recovered and cryopreserved to be stored by mothers and researchers in public and private cord blood banks (Riordan 2007).
BACKGROUND
Cord blood banking and transplantation has nearly a 30-year history with thousands of cord blood transplants having been performed in patients with hematopoietic malignancies, marrow failure and immunodeficiency disorders (Ballen, Gluckman, Broxmeyer 2013). Cells derived from umbilical cord blood have unique characteristics that enable regenerative medicine techniques and applications without the requirement to perfectly match tissue types (Riordan 2007). Umbilical cord blood contains hematopoietic stem cells (HSC), mesenchymal stem cells (MSC), and endothelial cells (Roura, Pujal, Galvez-Moon 2015). MSCs can help with inflammation modulatory response, release various growth factors, and can help supplement various connective tissue repair needs. Endothelial stem cells will form the lining of blood and lymph vessels and help vascularize tissue, making endothelial cells an increasingly compelling interest in the orthopedic applications (Atesok et al. 2012) and ischemic damage (Au et al. 2008). Cord blood stem cells are being observed for their trophic role, supplying growth factors, exosomes, cell-cell signaling, and immunomodulatory factors (Damien and Allan 2015; Rizk, Aziz, Shorr 2017). With these types of paracrine and autocrine effects on stimulating the body’s own ability to heal and regenerate (Rizk, Aziz, Shorr 2017), various applications are under wider investigation (orthopedic, neurological, immune modulation, etc.).
The uses of cord blood stem cells and growth factors in regenerative medicine are vast and emerging. The cord blood is recovered from live healthy birth donors of consenting mothers, then process and preserve the cord blood using a proprietary process to maximize the available biocellular and growth factor elements for safe allogeneic use. This Product Characterization
Report demonstrates the concentration, characterization, and quality of StemShot® – Umbilical Cord Blood Cellular Suspension Allograft.
METHODS & RESULTS
Umbilical Cord Blood Collection
Umbilical cord blood used in the StemShot® product is recovered from consenting mothers (donors) who deliver live-birth C-sections within a sterile operating room. Cord blood is collected at the time of birth via venipuncture of the umbilical vein and maintains sterility throughout the recovery process. All donors are screened and tested to ensure they meet the established FDA requirements for donating their post-natal birth tissue. An evaluation, performed by the manufacturer’s medical director, is performed to ensure there are no risks related to the donated birth tissue. The assessment includes a review of the donor’s medical history, health status, and personal behavior. A physical evaluation of the donor is also performed before delivery to ensure the mother is in good health. All donors are tested for infectious disease, as required by the FDA, including: HIV, HTLV, Hep B, Hep C, Syphilis, CMV, West Nile Virus, and Lyme disease. All post-natal birth tissue donations are identified with a unique donor identification number to ensure it can be tracked from the recovery / collection process and linked to the finished product for tracing, including storage and distribution.
Laboratory Processing
The donated umbilical cord blood is aseptically processed to ensure it is safe and effective for patient use. A sample from each manufactured lot, aligning with USP 71, is tested for sterility by a third-party lab. Additionally, aerobic and anaerobic testing is performed during manufacturing each lot to ensure the environment and product is free from bacteria, fungus, etc. The manufacturing process has been validated to remove 99.99% of red blood cells, while retaining the desirable cells and growth factors for optimal end product. The processed product is cryopreserved in a way to maximize the safe preservation of cellular and growth factor material into a fully suspended cryo state.
Cell Characterizations & Viability
3rd party testing – A sample of manufactured umbilical cord blood was tested by an independent third party, Franciscan Institute for Science and Health, for live nucleated cell counts and live cells. A rapid thawing protocol was used that aligns with the technique that the healthcare professional would use when administering the product. The protocol required the tester to hold the vial in a gloved hand until the frozen contents became liquid (under 5-minute thaw time). Measurements of the cell number, cell viability, and cell size were determined using a Nexcelom Cellometer (Figure 1). The total nucleated cells (TNC) was determined by staining with (AO/PI) acridine orange and the number of dead cells was determined by staining with propidium iodide. The viability rate or percent of viable cells was then calculated.
Figure 1 (From Exhibit A: Product Analysis Report)
DISCUSSION
The characteristics observed in the analysis of CytoShot® – Umbilical Cord Blood Cellular Suspension Allograft, show why cord blood stem cells are of growing interest in regenerative medicine. The allograft characteristics in cord blood evidence the distinct immunological benefits. Cord blood is rich in T regulatory cells, and cord blood cells have very little exposure to foreign antigens, making the T and B cells immunologically stable. The CytoShot® product contains millions of cells in a vial, as demonstrated by the AO/PI staining. Cord blood is positive for MSC and HSC markers, and cord blood has a strong record of safety (Vyas et al. 2014), making it an excellent choice by physicians for various regenerative medicine procedures.
DISCLAIMER
This product is exclusively intended for homologous use in humans. It may not be transferred to third parties, resold, modified or mixed for resale or used to manufacture commercial products for resale without written authorization from. There is no claim that treatment using this product is a cure for any condition, disease or injury.
CONFIDENTIALITY NOTICE
This document contains confidential and privileged information. If you know or suspect that you are not the intended recipient, please destroy all copies of this document. Any review, dissemination, use or copying of this message, attached documents, or of information contained within the message, by unauthorized or unintended recipients, is strictly prohibited.
Product Characterization Report: AmnioShotTM
Cytokines and growth factors contribute to the effects that amniotic fluid have on promoting the healing process.
INTRODUCTION
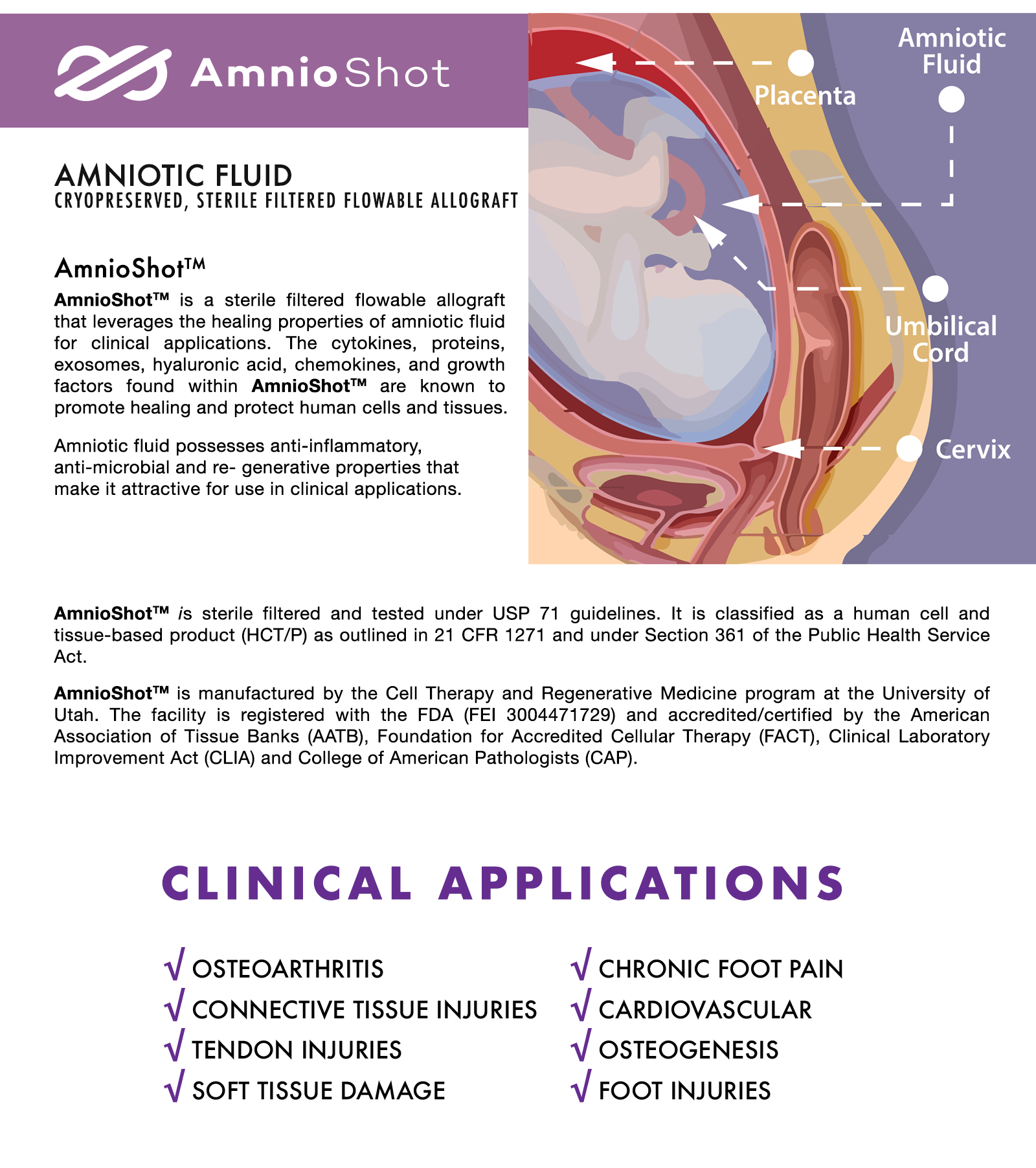
Early after conception and until the mother’s water breaks for the delivery of their infant, the fetus is bathed in amniotic fluid (AF). AF functions as a supportive cushion to the fetus and provides a protective environment. AF is a rich source of nutrients, cytokines and growth factors that are required for fetal development and maturation (Underwood et al. 2005). AF also contains stem cells with the potential to differentiate along multiple cell lineages (In’t Anker et al. 2003; Prusa et al. 2003; Bottai et al. 2012). The protective and regenerative properties of AF are achieved via the exchange of water and solutes with surrounding tissues. This is accomplished via the utilization of different pathways during the course of a pregnancy that likely contribute to changes in the composition of the AF with gestational age (Underwood et al. 2005).
BACKGROUND
Among some of the first evidence that AF has protective biological properties is a report describing that concentrates of AF inhibit the development of peritonitis (Johnson et al. 1936). This is followed by a report by Shimberg and co-workers that AF accelerates defense-repair mechanisms within damaged joints (Johnson et al. 1936; Shimberg 1938). Since these early publications, more sophisticated evaluations have revealed the presence of antimicrobial, immunomodulatory, and growth-promoting activities in AF (Underwood et al. 2005). Reports of antimicrobial activity in AF differ (Ismail et al. 1989) among investigators. Some studies show that AF is inhibitory, while others show no effect against the same microorganisms. Components with antimicrobial, antiviral and anti-fungal activity that are present in AF include lysozyme, peroxidase, transferrin, b -lysin, immunoglobulins and zinc-peptide complexes (Ismail et al. 1989). Immunomodulatory properties of AF are evident from studies showing that enteral feeding of AF suppresses the pro-inflammatory responses in preterm pigs with necrotizing enterocolitis (Siggers et al. 2013). While growth promoting activities of AF are supported by animal studies and by in vitro culture studies showing that AF can enhance neochondrogenesis (Ozgenel et al. 2004), regenerate peripheral nerves (Ozgenel and Filiz 2003) and bone (Karacal et al. 2005), accelerate reepithelialization in corneas (Castro-Combs et al. 2008), and promote healing of human skin wounds (Nyman et al. 2013). Some of the factors that are found in AF that may contribute to these activities include inflammatory mediators, but are not limited to TNF-a, IL-6, IL8, and IL-10 (Weissenbacher et al. 2012), trophic factors that include EGF, IGF-1, FGF, HGF and TGF-a (Merimee et al. 1984; Watanabe 1990; Lang and Searle 1994; Kurauchi et al. 1995; Hirai et al. 2002), and HA, an important factor in promoting reepithelialization in human skin wounds (Nyman et al. 2013).
Key Terms
Amniotic Fluid
Exosomes
Cytokines
Proteins & Growth Factors Hyaluronic Acid (HA)
ABSTRACT
Amniotic fluid (AF) possesses anti-inflammatory, anti-microbial and regenerative properties that make it attractive for use in clinical applications (Pierce et al. 2016).
The observations presented in the data and supporting literature support the conclusion that the cytokines and growth factors found in AmnioShotTM are likely to contribute to the effects that amniotic fluid have on promoting the healing process (Shimberg 1938; Colombo et al. 1993; Ozgenel et al. 2001, 2004; Castro-Combs et al. 2008; Ghaderi et al. 2011; Nyman et al. 2013; Feizi et al. 2014).
Regulatory Compliance
The AmnioShotTM product is manufactured by the University of Utah Cell Therapy and Regenerative Medicine. It is classified as a human tissue allograft (HCT/P) and meets the requirements for “Homologous Use” as outlined in 21 CFR 1271 under Section 361 of the Public Health Service Act. The sterile filtered amniotic fluid has no live cells, cell debris, hair, vernix or other debris, however it does contain human proteins, hyaluronic acid, cytokines, proteins and chemokines which are known to promote healing and protect human cells and tissues.
METHODS & RESULTS
Amniotic Fluid Collection
Human AF is collected from consenting mothers (donors) who deliver live-birth C-sections within a sterile operating room. A physician typically performs an abdominal fenistil incision through the abdominal and uterine muscles without cutting into the amnion membrane. Using a sterile soft suction catheter connected to a sterile collection system, a blunt end insertion with a catheter is made into the amnion membrane and the AF is aseptically suctioned into the container. The container is then labelled, wrapped in frozen Insul-ice mats and placed in a temperature monitored shipping container that is validated for transport between 2° C and 8° C. Upon arrival at the manufacturing facility, the product is immediately placed in a refrigerator until processing occurs.
Processing
AmnioShotTM is sterile filtered and tested under USP 71 guidelines. Each manufactured lot is tested to ensure there is no evidence of maternal blood contamination and that maternal blood samples test negative for infectious disease agents (i.e. HIV, HTLV, Hep B, Hep C, Syphilis, CMV, West Nile Virus).
Exosome Testing
A sample of AmnioShotTM product was tested by an independent laboratory, (The Saban Research Institute for Extracellular Vesicle Core Testing), to measure the total exosomes count and size. A Nanosight instrument was used and found a total exosome concentration count of 7.37×10^10, equivalent to 73 billion exosomes per milliliter, as highlighted in Exhibit A.
NANOSIGHTE9
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
AF100217-076 2019-02-27 13-46-56E9
Exhibit A – Exosome Testing Report
6.0
5.0
4.0
3.0
2.0
1.0
98
153
AF1~13-47-03 AF1~13-48-13 AF1~13-49-22 AF1~13-50-31 AF1~13-51-40
43
363
768
243
00
0
100 200 300 400 500 600 700 800 900 1000 0Size (nm)
FTLA Concentration / Size graph for Experiment: AF100217-076 2019-02-27 13-46-56
100 200 300 400 500 600 700 800 900 1000Size (nm)
15642 Sand Canyon Avenue, #50425, Irvine, CA 926181⁄2 Phone (888) 959-9635 1⁄2www.CellTherapyUSA.com
Averaged FTLA Concentration / Size for Experiment: AF100217-076 2019-02-27 13-46-56
Error bars indicate + / -1 standard error of the mean
|
Included Files AF100217-076 AF100217-076 AF100217-076 AF100217-076 AF100217-076 Details NTA Version: Script Used: Time Captured: Operator: Pre-treatment: Sample Name: Diluent: Remarks: Capture Settings Camera Type: Camera Level: Slider Shutter: Slider Gain: FPS Number of Frames: Temperature: Viscosity: Analysis Settings Detect Threshold: Blur Size: 2019-02-27 13-47-03 2019-02-27 13-48-13 2019-02-27 13-49-22 2019-02-27 13-50-31 2019-02-27 13-51-40 NTA 3.1 Build 3.1.46 sCMOS 25 7 |
Results Stats: Merged Data Stats: Mean +/- Standard Error 37.4 +/- 0.5 particles/frame 38.6 +/- 0.4 centres/frame |
DISCUSSION
The presented research, combined with the product characteristics data, identified within this report, demonstrate that nutrients, cytokines and growth factors contained in the non-cellular fraction of AF are useful for reparative and regenerative treatments in patients.
MANUFACTURER
AmnioShotTM is manufactured by the University of Utah Cell Therapy and Regenerative Medicine and is registered with the FDA (FEI 3004471729) and accredited or certified by the American Association of Tissue Banks (AATB), Foundation for Accredited Cellular Therapy (FACT), Clinical Laboratory Improvement Act (CLIA) and College of Pathologists (CAP).
DISCLAIMER
This product is exclusively intended for homologous use in humans. It may not be transferred to third parties, resold, modified or mixed for resale or used to manufacture commercial products for resale without written authorization from. There is no claim that treatment using this product is a cure for any condition, disease or injury.
CONFIDENTIALITY NOTICE
This document contains confidential and privileged information. If you know or suspect that you are not the intended recipient, please destroy all copies of this document. Any review, dissemination, use or copying of this message, attached documents, or of information contained within the message, by unauthorized or unintended recipients, is strictly prohibited.
Bottai D, Cigognini D, Nicora E, Moro M, Grimoldi MG, Adami R, Abrignani S, Marconi AM, Di Giulio AM, Gorio A (2012) Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub- populations. Restor Neurol Neurosci 30(1):55–68.
Castro-Combs J, Noguera G, Cano M, Yew M, Gehlbach PL, Palmer J, Behrens A (2008) Corneal wound healing is modulated by topical application of amniotic fluid in an ex vivo organ culture model. Exp Eye Res 87(1):56–63.
CFR-Title21 (2015). Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=1271&showFR=1&subpartNode=21:8.0. 1.5.57.3
Colombo JA, Napp M, Depaoli JR, Puissant V (1993) Trophic influences of human and rat amniotic fluid on neural tubederived rat fetal cells. Int J Dev Neurosci 11(3):347–355.
Feizi S, Soheili ZS, Bagheri A, Balagholi S, Mohammadian A, Rezaei-Kanavi M, Ahmadieh H, Samiei S, Negahban K (2014) Effect of amniotic fluid on the in vitro culture of human corneal endothelial cells. Exp Eye Res122:132–140.
Ghaderi S, Soheili ZS, Ahmadieh H, Davari M, Jahromi FS, Samie S, Rezaie-Kanavi M, Pakravesh J, Deezagi A (2011) Human amniotic fluid promotes retinal pigmented epithelial cells’ trans-differentiation into rod photoreceptors and retinal ganglion cells. Stem Cells Dev 20(9):1615–1625.
Hirai C, Ichiba H, Saito M, Shintaku H, Yamano T, Kusuda S (2002) Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J Pediatr Gastroenterol Nutr 34(5):524–528.
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102(4): 1548–1549.
Ismail MA, Salti GI, Moawad AH (1989) Effect of amniotic fluid on bacterial recovery and growth: clinical implications. Obstet Gynecol Surv 44(8):571–577.
Johnson HL, Hazard JB, Foisee PS, Aufranc O (1936) Amniotic fluid concentrate as an activator of peritoneal immunity. Surg Bynec Ostet 62:171–181.
Karacal N, Kosucu P, Cobanglu U, Kutlu N (2005) Effect of human amniotic fluid on bone healing. J Surg Res 129(2): 283–287. Kurauchi O, Itakura A, Ando H, Kuno N, Mizutani S, Tomoda Y (1995) The concentration of hepatocyte growth factor (HGF) in
human amniotic fluid at second trimester: relation to fetal birth weight. Horm Metab Res 27(7):335–338.
Lang AK, Searle RF (1994) The immunomodulatory activity of human amniotic fluid can be correlated with transforming growth
factor-beta 1 (TGF-beta 1) and beta 2 activity. Clin Exp Immunol 97(1):158–163.
Merimee TJ, Grant M, Tyson JE (1984) Insulin-like growth factors in amniotic fluid. J Clin Endocrinol Metab 59(4):752–755. Nyman E, Huss F, Nyman T, Junker J, Kratz G (2013) Hyaluronic acid, an important factor in the wound healing properties of
amniotic fluid: in vitro studies of re-epithelialisation in human skin wounds. J Plast Surg Hand Surg 47(2):89–92. Int J
Gynaecol Obstet 24(2):97–101.
Ozgenel GY, Filiz G (2003) Effects of human amniotic fluid on peripheral nerve scarring and regeneration in rats. J Neurosurg
98(2):371–377.
Ozgenel GY, Samli B, Ozcan M (2001) Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing
after flexor tendon surgery in rabbits. J Hand Surg Am 26(2):332–339.
Ozgenel GY, Filiz G, Ozcan M (2004) Effects of human amniotic fluid on cartilage regeneration from free perichondrial grafts in
rabbits. Br J Plast Surg 57(5):423–428.
Pierce J, Jacobson P, Benedetti E, Peterson E, Phibbs J, Preslar A, Reems J, Collection and characterization of amniotic fluid
from scheduled C-section deliveries. Cell and Tissue Banking, International Journal for Banking, Engineering and
Transplantation of Cells and Tissues Incorporating Advances in Tissue Banking, ISSN 1389-9333 (2016).
Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M (2003) Oct-4-expressing cells in human amniotic fluid: a new
source for stemcell research?HumReprod 18(7):1489–1493.
Shimberg M (1938) The use of amniotic fluid concentrate in orthopaedic conditions. J Bone Joint Surg Am 20(1): 167–177. Siggers J, Ostergaard MV, Siggers RH, Skovgaard K, Molbak L, Thymann T, Schmidt M, Moller HK, Purup S, Fink LN,
Frokiaer H, Boye M, Sangild PT, Bering SB (2013) Postnatal amniotic fluid intake reduces gut inflammatory responses and
necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol 304(10): G864–G875.
Underwood MA, Gilbert WM, Sherman MP (2005) Amniotic fluid: not just fetal urine anymore. J Perinatol 25(5): 341–348. Watanabe H (1990) Epidermal growth factor in urine of pregnant women and in amniotic fluid throughout pregnancy. Gynecol
Endocrinol 4(1):43–50.
Weissenbacher T, Laubender RP, Witkin SS, Gingelmaier A, Schiessl B, Kainer F, Friese K, Jeschke U, Dian D, Karl K (2012)
Influence of maternal age, gestational age and fetal gender on expression of immune mediators in amniotic fluid. BMC Res Notes 5:375.
Medical Professional Viewing Only (Disclaimer)
This site was intended for education purposes only and strictly for use by medical professionals. The FDA recently re-confirmed, there is only one registered stem cell product, and while there is enormous promise in stem cell therapies, and thousands of ongoing experimental applications trying to establish efficacy, these are not at the point where they would meet the scientific standard.
The FDA has stated:
Stem cells, like other medical products that are intended to treat, cure or prevent disease, generally require FDA approval before they can be marketed. FDA has not approved any stem cell-based products for use, other than cord blood-derived hematopoietic progenitor cells (blood forming stem cells) for certain indications.
http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194655.htm
This site is not intended for consumers.
If you are considering stem cell treatment in the U.S., ask your physician if the necessary FDA approval has been obtained or if you will be part of an FDA-regulated clinical study. This also applies if the stem cells are your own. Even if the cells are yours, there are safety risks, including risks introduced when the cells are manipulated after removal.
“There is a potential safety risk when you put cells in an area where they are not performing the same biological function as they were when in their original location in the body.” Cells in a different environment may multiply, form tumors, or may leave the site you put them in and migrate somewhere else.
If you are considering having stem cell treatment in another country, learn all you can about regulations covering the products in that country. Exercise caution before undergoing treatment with a stem cell-based product in a country that—unlike the U.S.—may not require clinical studies designed to demonstrate that the product is safe and effective. FDA does not regulate stem cell treatments used solely in countries other than the United States and typically has little information about foreign establishments or their stem cell products.
http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm
Stem cell therapies have enormous promise, but the science in each use is still in the developmental stage. Professional judgment and expertise is needed in using stem cells for any therapeutic use, and we urge anyone embarking on the use of stem cell therapies to consult the national health data bases to evaluate current information from clinical trials and the FDA websites on human tissue should also be consulted to get its current evaluation of any therapy.
Orders@CellTherapyUSA.com
Phone: (888) 959-9635 | Fax: (877) 695-2048 | www.CellTherapyUSA.com
